Tuberous sclerosis complex (TSC) is a genetic multisystemic disease that affects approximately 1 in 6,000 live births.1 It causes noncancerous tumors, or hamartomas, to form throughout the body. These benign tumors can develop in many organs, including the brain, eyes, kidneys, liver, lungs, heart, and skin.1
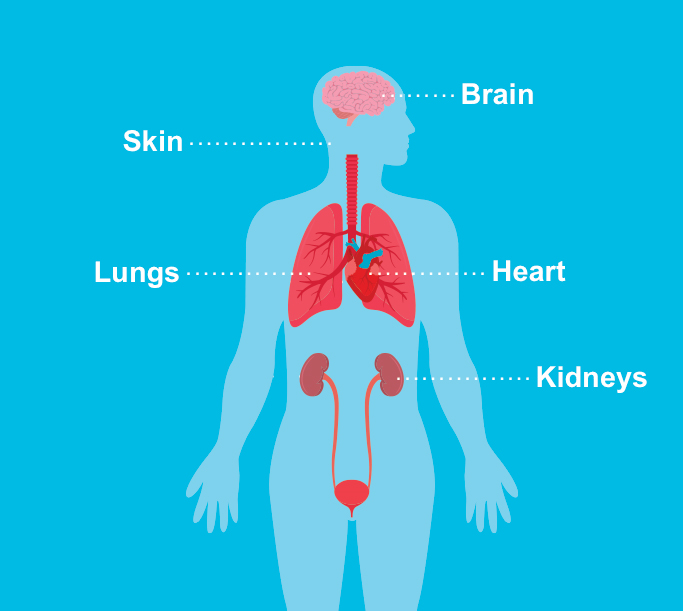
The skin is one of the most affected organs in people with TSC.3
Facial angiofibromas are pink-to-red tumors typically located on the cheeks, nose, and chin, often appearing in a butterfly pattern over the malar eminences and nasolabial folds.1,4
Facial angiofibromas are made up of blood vessels and fibrous tissue and can coalesce to form patches. They can bleed, obstruct the nasal openings, and cause disfigurement.3,5
Facial angiofibroma occurs in approximately 75%-80% of TSC patients, making it the one of the most predominant skin manifestations of this disease.3-5
These tumors are sometimes treated with invasive modalities. However, these procedures may require anesthesia. Invasive treatments can also be difficult to administer in patients with extensive angiofibroma or severe intellectual impairments.4
CONTRAINDICATIONS
HYFTOR® is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of HYFTOR®.
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (≥1%) are dry skin, application site irritation, pruritus, acne, acneiform dermatitis, ocular hyperemia, skin hemorrhage, and skin irritation.
USE IN SPECIFIC POPULATIONS
Please see full Prescribing Information for additional safety information.
HYFTOR® is an mTOR inhibitor immunosuppressant indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients 6 years of age and older.
CONTRAINDICATIONS
HYFTOR® is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of HYFTOR®.
This site is intended only for U.S. healthcare professionals. The products discussed in this site may have different product labeling in different countries. The information provided is for educational purposes only.
This site is intended for U.S. healthcare professionals only.
By submitting your email address, you are opting in and may be provided with online advertisements or communications on Nobelpharma or third-party websites that are tailored to you, for example, on the basis of nonidentifying information or of your web browsing activity. When a Nobelpharma communication is delivered to you, you will see information about how to opt-out. By clicking the arrow, you agree and understand that your information will be governed by our Privacy Policy.
Link pending once FDA approval comes in…